Abstract
INTRODUCTION The treatment of Low and Intermediate risk Acute Promyelocytic Leukemia (APML) with Arsenic Trioxide (ATO) and All Trans Retinoic Acid (ATRA) has become the standard of care. However, most protocols include the addition of chemotherapy for high-risk patients. We describe our experience of treating APML patients of all risk classes with ATO and ATRA without chemotherapeutic agents.
METHODS All patients diagnosed with APML with RTPCR positive for PML-RARA from 2006-2021 were included in the study and their records were retrospectively reviewed. Patients were classified according to the Sanz risk classification into low, intermediate and high risk. The follow-up cut-off date was 28/2/2022, 1 year after the last patient was included in the study. Patients with early mortality (defined as death within 7 days of therapy initiation) were excluded from the safety and efficacy analysis. The per-protocol population refers to the patients who did not die within 7 days of initiation of therapy.
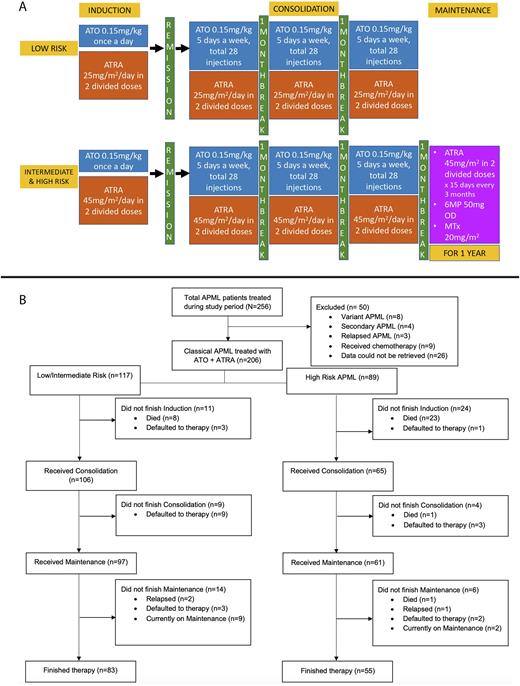
Study Protocol (Figure 1A)
All patients were started on ATO and ATRA on the first day of therapy. Patients received ATO for at least 28 injections or till the achievement of complete hematological response (CHR) (whichever was later). Hydroxyurea was given for cytoreduction (up to 2gm q2hourly) on physician discretion if the TLC>5x109/L. A bone marrow with RTPCR was done after induction to document response, after which ATRA was stopped. Patients received 3 cycles of consolidation with ATO and ATRA (each one month apart) after achieving morphological remission. Patients with Intermediate and High-risk disease received 2 years of maintenance with ATRA for 15 days once every 3 months, daily 6-Mercaptopurine and weekly oral Methotrexate. After a protocol modification in 2015, oral Prednisolone (0.5-1mg/kg/day for 14 days followed by taper) was added to the induction therapy and maintenance therapy was reduced to 1 year. We wish to acknowledge CHEARTC for providing our patients with ATO and ATRA free of cost
RESULTS Two hundred fifty-six patients with APML were treated at our centre during the study period, of whom 206 patients were included in the study for analysis (Figure 1B). The median age was 32 years with a Male to Female ratio of 1.3:1. The median duration of symptoms was 24 days (Range 2-180). At presentation, 157 patients (76.2%) had fever, and 169 (82%) had bleeding manifestations. The most common site of bleed was epistaxis and gum bleeds (111; 53.9%); 24 patients (11.7%) had a major bleed and 6 patients (2.9%) had thrombosis at presentation. Most of the patients were Intermediate risk (N=107; 51.9%) followed by High risk (N=89, 43.2%). Differentiation syndrome (DS) was seen in 41 patients (19.9%) and was more common in high-risk patients (32.4% vs 11.8%, p-0.001). The incidence of DS was lower in patients who received prophylactic steroids after a protocol modification in 2015 (13.3% vs 35.2%, p-0.001).
Twenty-five patients (12.1%) died within 7 days of initiating therapy, and the rest of the analysis was done on the remaining patients (N=181). The median number of days to achieve CHR was 34 (Range 16-65). Eighty-three patients (45.9%) had an infection during induction, with the most common site being pulmonary (N=44; 53%).
Four patients defaulted during induction, and 6 patients died during induction in the per-protocol population. Of the 171 patients who underwent a marrow after induction, 157 were in remission. The other 14 patients received 2 additional weeks of induction, and all achieved remission after extended therapy. One thirty-three patients (77.7%) were PML RARA PCR negative at the end of induction, and only 4 patients were PCR positive at the end of consolidation.
The median follow-up for the per-protocol population was 45 months (Range 0-186). Seven patients relapsed during follow up- 3 during maintenance, and 4 after completion of therapy. Thirteen patients in the per-protocol population died during follow-up. The most common cause of death was infection (seen in 6 patients). Median EFS and OS were not reached for the study population. The estimated 5-year EFS and OS in the per-protocol population were 90 ± 5.8% and 93 ± 3.9%, respectively. The estimated 5-year EFS and OS in the entire cohort were 79 ± 5.8% and 80 ± 5.8%, respectively.
CONCLUSION Treatment of all risk classes of APML with ATO and ATRA without chemotherapy is associated with excellent long-term outcomes in the real-world setting.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal